Research
1. Aqueous Lithium-Oxygen Batteries
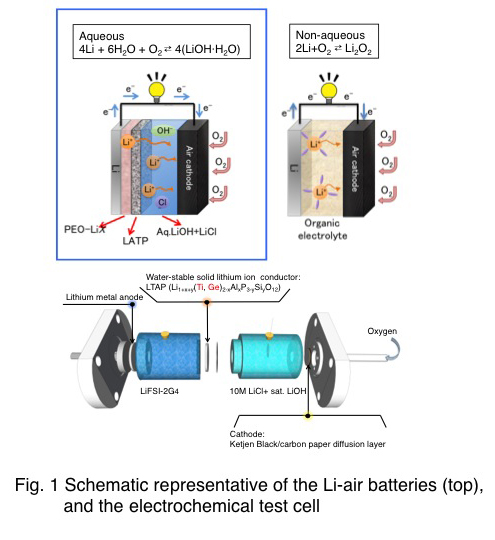
The electrochemical process of an aqueous Li-O2 cell is investigated. A Li2O2 is detected as a discharging product of an aqueous Li-O2 cell using a catalyst-free carbon-based electrode. The electrolyte solution saturated with lithium hydroxide prevents the hydrolysis of the Li2O2. Since the electron transfer process is based on the oxygen-peroxide redox couple, the galvanostatic charging-discharging profile shows stable cycling with extremely low charging overpotential < 0.1 V at 1.0 mAcm-2.
2. Garnet-type Lithium Ion Conductor Li7La3Zr2O12
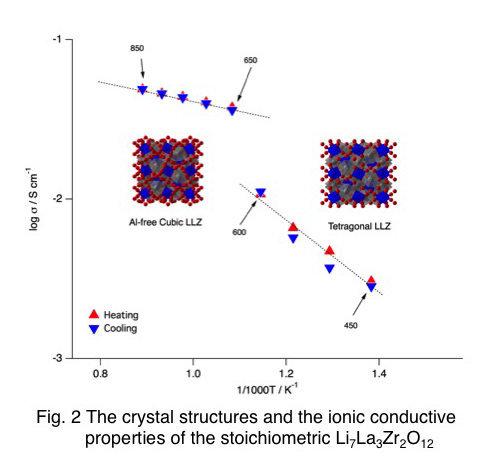
Our group has been investigated Garnet-type lithium-ion conductor Li7La3Zr2O12 (LLZ) and its related compounds as an electrolyte for lithium secondary batteries and a protective layer for a water-stable lithium electrode for lithium-air rechargeable batteries. Because these compounds exhibit ≥ 3 x 10−4 S cm−1 of conductivity at 25ºC in addition to chemical and electrochemical stability with lithium metal. Three phases have been reported for LLZ; a high temperature (H.T.)-cubic phase which forms a disordered lithium arrangement, a tetragonal phase obtained whose lithium sites are completely ordered with 100 % occupancy, and a low temperature (L.T.)-cubic phase formed by reaction with moisture and/or CO2. Among these phases, the H.T.-cubic phase is the most popular one owing to its high ionic conductivity.
We investigated the phase stability of LLZ using high temperature X-ray diffraction (H.T.-XRD). The thermodynamically stable phase of the stoichiometric LLZ at room temperature is the tetragonal phase and it transformed into the non-quenchable cubic phase at around 650°C. The H.T.-cubic phase was stabilized at room temperature by the introduction of the lithium vacancies. The formation of the L.T.-cubic phase at around 200ºC is strongly affected by the Li+/H+ exchange reaction with moisture.
3. High Voltage Spinel Cathode LiNi1/2Mn3/2O4
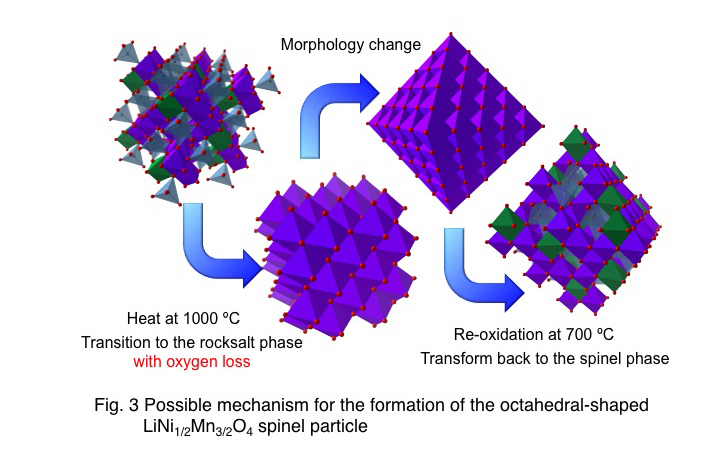
Spinel-type LiNi1/2Mn3/2O4 is one of the promising cathode materials for high-voltage lithium ion batteries. The biggest challenge of this material is the capacity degregation due to the decomposition of the electrolyte. However a significant improvement of the cycle stability was acheved by the highliy crystallined LiNi1/2Mn3/2O4 cathode.
We also successfully synthesized the highly crystallined LiNi1/2Mn3/2O4 cathode and demonstrated < 95 % of capacity retention after 100 cycles at 60ºC. However, the formation mechanism of highly crystallined spinel oxides is not well understood yet. Therefore, we have been investigated the crystal growth process of this material and sucseeded the morphology controled LiNi1/2Mn3/2O4 with small particle by a low temperature synthesis.
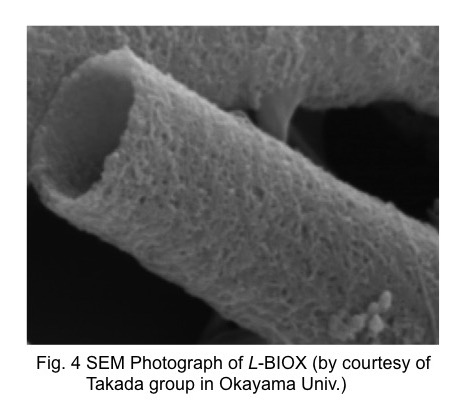
4. Nanometric ion oxide of bacterial origin as a Conversion anode for LIB
A species of aquatic iron-oxidizing bacteria living worldwide produces extracellular microtubules composed of loosely connected Fe-rich amorphous oxide particles (Fe:Si:P~73:22:5, ~3 nm across), which the Takada’s group(Okayama Univ.) found and calls BIOX. We collaborate with the group on the application as an Fe3+/Fe0 conversion anode material for Li-ion batteries.
The BIOX has a microtubular form with a fixed bore diameter of ~1 μm and variable lengths up to several centimeters, whose structure consists of primary particles of ~3 nm across. The Si and P included share the formation of structure and prevent the grain growth during the cycling. The unique texture brings the high capacity as well as the excellent cycle and rate performances.
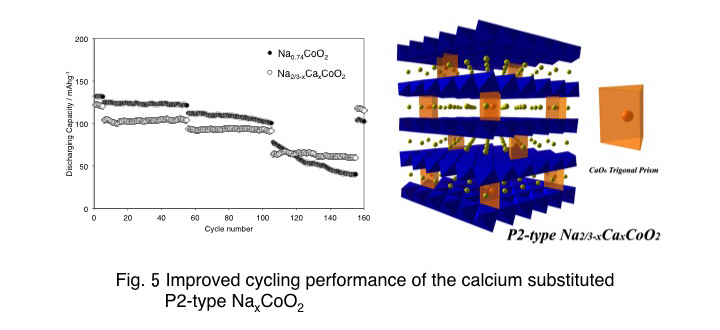
5. Cathode Active Materials for Na-ion Batteries
In recent years, Na-ion batteries are expected as a potential post Li-ion battery system because of the abundant resources. A lot of layered materials: NaxMO2 (M=Cr, Mn, Fe, Co, Ni) have been investigated as candidate positive electrode materials for Na-ion batteries. Substitution of the transition metal in NaxMO2 is common strategy for the improvement of the capacity and the cycling performances. On the other hand, elemental substitution of Na sites has never been investigated because the substituted atoms easily hinder the smooth diffusion of Na+ in the active materials. In this study, we intentionally performed partial substitution of Na+ sites in NaxCoO2 with Ca2+ and investigated how it affected to the electrochemical properties. Even though the partial substitution of Na+ sites with Ca2+ decreases the specific capacity and increases the over potential, the cycling performance of the electrode at high charge-discharge current showed significant improvement. The results suggested that the substituted Ca2+ stabilized the layered structure and eliminated the irreversible phase-transformation.